ISO 9626 provides critical guidelines for testing stainless steel needle tubing used in medical devices, such as hypodermic needles. These tests, including the preskus togosti igelnih cevi and breakage resistance, ensure that the tubing maintains mechanical integrity under various conditions, contributing to the safety and effectiveness of medical devices. This article explains the key aspects of ISO 9626, je preskus togosti igelnih cevi, and related tests such as corrosion resistance in . odpornost proti zlomu igelnih cevi.
ISO 9626:2016 specifies the requirements for rigid stainless steel needle tubing used in the production of medical devices, primarily for human use. The standard applies to tubing of various metric sizes, ranging from 3.4 mm (10 Gauge) na . 0.18 mm (34 Gauge). It covers mechanical properties such as needle tubing stiffness, breakage resistance, and corrosion resistance, which are crucial for ensuring the safety and reliability of medical devices.
ISO 9626 outlines the testing methods for determining the physical properties of needle tubing, including tensile strength, stiffnessin breakage resistance. These tests are essential for manufacturers to comply with industry standards and provide high-quality, durable products.
Spletna stran preskus togosti igelnih cevi is an integral part of ISO 9626. This test evaluates the ability of the tubing to resist bending under force. To perform the test, a specified force is applied to the center of a given length of tubing, which is supported at both ends. The amount of deflection is then measured.
The stiffness test requires a stiffness testing apparatus capable of applying a downward force of up to 60 N. This apparatus should have a plunger with a blunt wedge at the bottom, and the force applied should be measured with accuracy of ±0.1 N. The deflection of the tubing should be measured to the nearest 0,01 mm.
The test provides important data for understanding how a needle’s tubing will perform during use, including its flexibility and ability to withstand external forces without failure. Manufacturers rely on the stiffness test to ensure that their products meet the necessary mechanical standards.
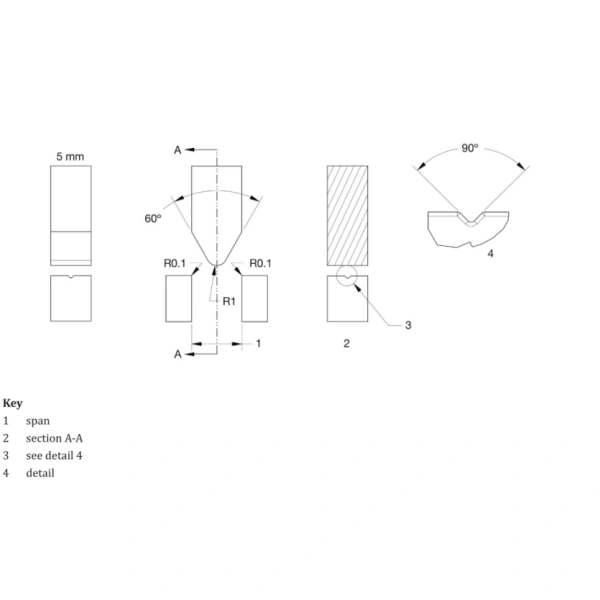
Another key test specified in ISO 9626 is the needle tubing breakage resistance test. This test evaluates the ability of needle tubing to withstand repeated bending without breaking, which is critical in applications where the needle may be subjected to stress during insertion.
The test involves applying a force to the tubing, bending it through a specified angle and repeating the bending cycles. The tubing must withstand 20 cycles of bending in both directions without breaking. This test simulates the forces the tubing might encounter during routine use and ensures that the needle will not fail under normal condition
Spletna stran corrosion resistance of needle tubing is another crucial property, particularly for medical devices exposed to various chemicals and sterilization processes. ISO 9626 outlines a method for testing corrosion resistance by immersing the tubing in a sodium chloride solution and observing the effects after a set period.
The test involves immersing the tubing in a 0.5 mol/l sodium chloride solution at 23 ± 2°C za 7 hours. After the immersion period, the tubing is inspected for any signs of corrosion. Corrosion resistance is essential for ensuring the longevity and integrity of the needle tubing, particularly in environments where it is exposed to saline solutions or sterilizing agents.


Izvajanje natančnih needle tubing stiffness tests, it is essential to use the correct equipment. The Syringe Tube Stiffness Tester je zasnovan za merjenje bending resistance of needle tubing, ensuring that the product meets the necessary standards for use in medical applications. This instrument can measure the deflection of the tubing under varying loads, allowing manufacturers to assess the quality of the tubing and ensure its durability.
The Syringe Tube Stiffness Tester is highly accurate and provides reliable data for manufacturers to ensure compliance with ISO 9626 standards. Using this equipment helps guarantee that needle tubing is stiff enough to maintain its shape and integrity during use but flexible enough to be inserted easily.
By following ISO 9626 and using appropriate testing equipment, manufacturers can produce durable and high-quality needle tubing that ensures the safety of patients and the efficacy of medical devices.
Syringetest.com zagotavlja napredne oprema za testiranje brizg designed to support complete compliance and streamlined QC workflows. If you’re looking to upgrade your syringe validation process or improve test accuracy, contact us to learn how our instruments can help meet your standards.
