ISO 7886-1 is the internationally recognized standard that defines the performance and testing requirements for sterile hypodermic syringes for single use, intended for manual operation. This standard plays a vital role in syringe quality control, ensuring functional safety and patient protection. Quality control engineers and regulatory specialists rely on this document to guide syringe functionality testing and validate product performance. From break loose and glide force testing to syringe leak testing, ISO 7886-1 outlines precise test methods for evaluating syringe integrity.
To reduce medication waste and minimize the risk of cross-contamination, ISO 7886-1 requires that syringe dead space—the residual liquid volume remaining after full plunger insertion—must be kept to a minimum.
Syringes must demonstrate robust sealing performance. According to Annex D, there should be no visible leakage of water past the plunger stopper or seal under compression. While small droplets between internal seals are permissible, external leakage constitutes a failure. Additionally, Annex B mandates that no air leakage or drop in vacuum pressure may occur during aspiration testing. These criteria ensure the syringe’s barrier integrity and safe clinical application.
ISO 7886-1 recommends verifying the forces required to initiate and sustain plunger movement, as syringe usability and dose control depend on it. Annex E presents a method to measure the break loose and glide forces using a mechanical testing system. Although this test is informative rather than mandatory, it plays a crucial role in ensuring consistent and user-friendly performance.
The plunger stopper must fit securely within the barrel. To confirm this, the syringe is filled to its nominal capacity and held vertically—both upright and inverted. In either orientation, the plunger should not move due to its own weight or the weight of the water alone. This requirement safeguards against unintended movement that could result in dosing errors or leakage.
Annex E of ISO 7886-1 specifies the method for determining the break loose and glide force required to operate the plunger. These forces influence the user experience and dosage control.
Test Method Highlights:
Consistent glide performance ensures injection smoothness and user comfort, making it a critical quality benchmark.
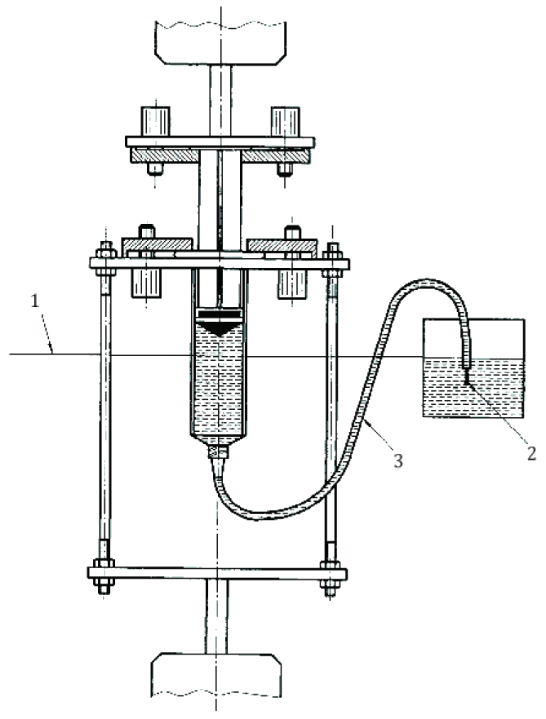
Annex D outlines a method to detect liquid leakage past the plunger stopper seal during compression—a crucial indicator of syringe reliability.
Test Process:
Cell Instruments offers the SLT-02 Syringe Leak Tester, ideal for precise pressure control and real-time observation.
Annex B defines procedures for air leak detection of syringe and verifying plunger stopper retention during aspiration.
Procedure:
Air tightness ensures dosage accuracy and prevents contamination—making this test essential for safe use.


This instrument precisely applies a controlled vacuum to the syringe, enabling accurate monitoring of pressure changes and plunger integrity. Designed with ISO 80369-7-compatible connections and secure clamping mechanisms, the SLT-01 ensures thorough evaluation of both air tightness and plunger stopper retention.

It utilizes a high-precision mechanical system to measure break loose and glide forces during fluid expulsion. The MST-01 enables manufacturers to quantify initiation and travel resistance, providing essential data to ensure smooth and consistent plunger motion—critical for safe and comfortable.

This device simulates pressure inside the syringe barrel while applying lateral and axial loads to the plunger, closely replicating clinical use conditions. The SSPT-01 is engineered for detecting even subtle seal failures, helping manufacturers verify product reliability and compliance with stringent safety standards.
Complying with ISO 7886-1 ensures that syringes:
Neglecting proper syringe functionality testing can lead to product recalls, regulatory penalties, or patient harm.
Understanding and applying ISO 7886-1 testing methods is essential for any company involved in syringe production or quality control. From break loose and glide force testing to air and liquid leak detection, each test serves a specific role in safeguarding product reliability and user safety.
Syringetest.com provides advanced syringe testing equipment designed to support complete compliance and streamlined QC workflows. If you’re looking to upgrade your syringe validation process or improve test accuracy, contact us to learn how our instruments can help meet your standards.
