Understanding luer lock adaptor collar torque resistance is critical for ensuring the quality and reliability of prefilled syringe assemblies. This test verifies the ability of the Luer Lock Adaptor (LLA) collar to resist rotational forces when a Luer fitting, such as a needle hub, is attached. Ensuring that your syringes meet performance standards is not just about compliance—it is about protecting end users and maintaining your brand’s reputation.
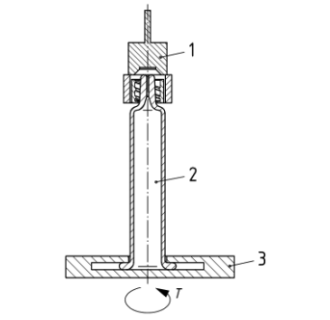
The luer lock adaptor collar torque resistance test evaluates the mechanical stability of the syringe’s LLA collar under torsional stress. If the collar fails during or after needle attachment, it could result in serious consequences such as leakage, dosing errors, or even injury.
By performing this test, manufacturers can ensure:
Testing this property is especially vital for sterilized subassembled syringes ready for filling, ensuring that the product maintains its integrity from manufacturing to administration.
The ISO 11040-4 Annex G4 section specifically outlines how to conduct luer lock adaptor collar torque resistance tests in a consistent and standardized manner. Here’s an overview of the essentials:
The method assesses whether the LLA collar can withstand a rotational force applied during the connection of a Luer fitting. The collar must remain secure without excessive movement or detachment.
It’s important to use calibrated equipment to ensure the accuracy of the measurements. Syringetest.com’s torque testing solutions provide high sampling rates and reliable data acquisition to meet these needs.
Test samples should be handled under the same conditions as the final delivered product. Consistency ensures that the test results accurately reflect real-world performance.
Performing a luer lock adaptor collar torque resistance test according to ISO 11040-4 Annex G4 involves the following steps:
The maximum recorded torque represents the collar’s resistance to rotational force and must meet the specifications defined in the product standards.
After completing the test, it is crucial to document:
Traceability is essential. Even if some setup parameters are not printed automatically, they should be logged manually to maintain full traceability.
At Syringetest.com, we understand that reliable luer lock adaptor collar torque resistance testing is essential for your syringe products’ success. We offer high-precision torque testers that meet or exceed the requirements of ISO 11040-4 and ISO 11040-4 Annex G4. Our instruments feature:
Moreover, we support our customers with technical expertise and automation customization, ensuring that your testing workflows are efficient, accurate, and compliant.
Testing luer lock adaptor collar torque resistance according to ISO 11040-4 Annex G4 is not merely a regulatory formality—it is a critical quality control step that ensures product safety, reliability, and user satisfaction. With the right equipment and testing methodology, you can confidently bring safe and compliant syringe products to market.
If you need assistance with setting up your torque resistance tests or selecting the right testing instruments, Syringetest.com is here to help. Contact us today to learn more about our solutions tailored to your syringe testing needs.
