In the pharmaceutical and medical industries, the pull-off force of the tip cap or the needle shield plays a vital role in ensuring the integrity, usability, and safety of prefilled syringes. Accurate measurement and control of this parameter directly affect product reliability and patient safety. For professionals involved in syringe quality control, understanding how to correctly evaluate pull-off force — in alignment with ISO 11040-4 and ISO 11040-4 Annex G5 standards — is essential.
The pull-off force of the tip cap or the needle shield refers to the amount of force required to remove the protective cover from the syringe tip or needle before use.
A properly calibrated pull-off force ensures:
If the pull-off force is too low, it may compromise product safety. Conversely, if it is too high, it can hinder usability, especially in clinical settings where speed and ease are critical.
International standards such as ISO 11040-4 and particularly ISO 11040-4 Annex G5 provide detailed methodologies to evaluate critical components of prefilled syringes, including the tip cap and needle shield.
These standards establish:
By adhering to these protocols, manufacturers and quality inspection agencies ensure consistent, reproducible, and internationally accepted results. Syringetest.com specializes in providing precision testing equipment compliant with these standards, ensuring optimal performance for your syringe evaluations.
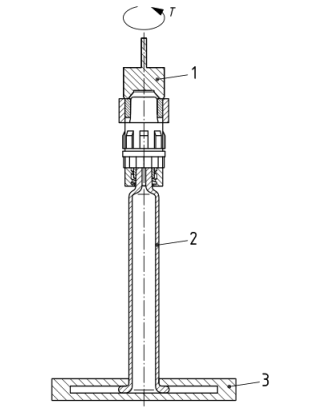

For precise testing of the pull-off force of the tip cap or the needle shield, specialized testing equipment is required. A typical setup includes:
At Syringetest.com, we offer high-accuracy pull-off force testers configured specifically to meet ISO 11040-4 Annex G5 requirements.
The testing process involves several critical steps:
Following the above practices guarantees reliable results, aligns with ISO 11040-4 Annex G5, and strengthens product compliance and patient safety assurances.
Testing the pull-off force of the tip cap or the needle shield is crucial for:
By ensuring correct pull-off forces, manufacturers enhance product usability, patient satisfaction, and regulatory acceptance — directly contributing to brand reliability and market success.
Syringetest.com provides state-of-the-art, customizable testing solutions to meet diverse syringe and medical device testing needs. Our instruments are designed to support stringent quality demands across packaging, pharmaceutical, and healthcare sectors.
Understanding and accurately measuring the pull-off force of the tip cap or the needle shield, guided by standards such as ISO 11040-4 and ISO 11040-4 Annex G5, is vital for ensuring the safety, usability, and regulatory compliance of prefilled syringes. Relying on precision instruments from Syringetest.com ensures your testing is efficient, accurate, and in full alignment with global standards.
